Filling Out The Data Access Agreement
Last updated: 2 years ago (view history), Time to read: 7 minsThe Data Access Agreement (DAA) is a legal document used by St. Jude Cloud to verify the identity and intent of those requesting to access St. Jude Children’s Research Hospital’s genomics data. The document binds you and your institution in agreement to protect, use and share the data appropriately.
Upon selection of your desired data, you will be prompted to complete a Data Access Agreement if you have not already been approved for access to the selected datasets. In order to simplify the data access request process, an electronic data access agreement is available for US residents only.
If you reside outside of the US, you must fill out the Data Access Agreement manually. You may click here to download the latest version of the DAA. Please read the first 6 pages carefully, which consist of terms and conditions that you and your institution must agree to in order to access any data on St. Jude Cloud. Then, follow the directions starting at Data Access Units to ensure that you have correctly filled out the DAA. Please note that there are two additional required sections if you intend to download data to your local infrastructure.
The Data Access Agreement
Downloading Data
If and only if you wish to download the genomic data locally, you must have the Principal Investigator initial on page 9 and have the Information Security Officer sign on page 15. If filling out the Data Access Agreement electronically, you must select the option to download within the setup wizard and input the contact information of your institution’s Information Security Officer.
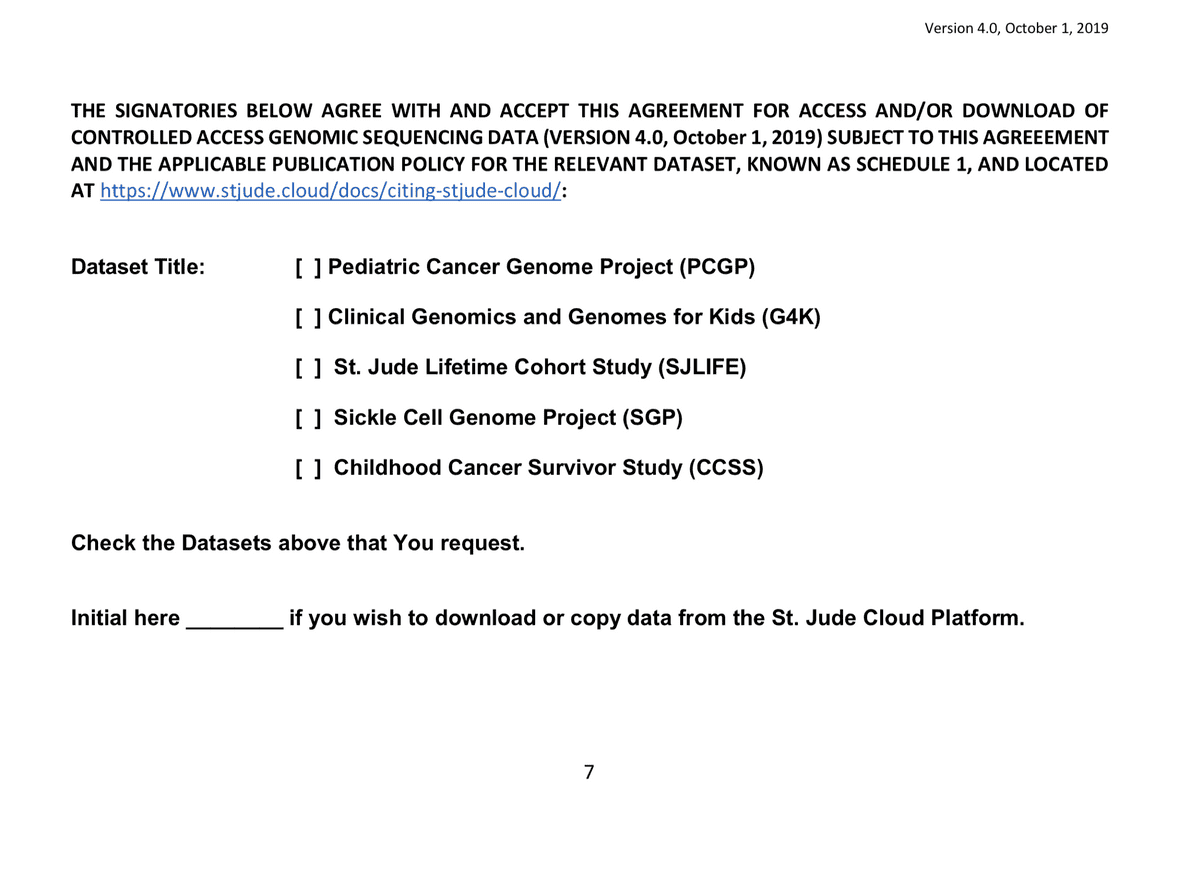
Data Access Units
A St. Jude Cloud Data Access Unit (DAU) is a grouping of data that typically corresponds to a project, study, or dataset generated at the same time at the same institution. To learn more, see our section on Data Access Units.
On page 9 of the DAA, you must mark all Data Access Units for which you are applying. The DAU(s) associated to the data you requested are listed in the Controlled Access Data section, directly above the Download Data Access Agreement button. This can be found on the Request Data webpage which immediately follows your selection of data from the data browser.
If you mark the incorrect datasets, you will be required to resubmit your agreement with the correct datasets marked. When completing the DAA electronically using the setup wizard, the DAU options will be automatically preselected for you.
Contemplated Use
On page 10 of the DAA, you must submit a description of your research project. Please specifically describe the intended role of St. Jude’s data in your research project. Your contemplated use can be anywhere from a paragraph to a few pages long, although a typical contemplated use is 1-2 paragraphs. Each Data Access Committee will evaluate your contemplated use case and decide whether to approve or deny your application for data access based on their own set of protocols. Please contact us if you have any questions regarding the protocols of the approval process.
Contemplated Use Example
The below is a simulated example of a contemplated use.
“We propose to apply our own variant calling method to detect structural variants in both pediatric tumor and germline samples. The detected variants will then be compared to our own genomic data and to publicly available data sources of normal and disease samples to assess the novelty of those variants and their association with disease. Variants will be categorized by their expected effect on genes known to be relevant to cancer, DNA repair, or epigenetics. One goal of the research is to compare our mutation detection software to existing methods on samples with known mutations and structural variants such as those in the PCGP data set. Another goal is the identification of novel variants with potential clinical relevance. Promising tractable variants will be introduced into cell lines to observe their effect on cell growth and tumor progression. Lastly, we propose to use the results of our analyses to further develop and refine our variant detection methods.”
Principal Investigator
On page 11 of the DAA, the Principal Investigator of the research project must input their information and sign the agreement. Typically the PI signee is the faculty-level supervisor on the project, but it is not a requirement.
Who may qualify as a Principal Investigator (PI)?
The PI is designated by the grantee organization to direct the project or activity being supported by the grant. The PI is responsible and accountable to the grantee for the proper conduct of the project or activity. The role of the PI within the eRA Commons is to complete the grant process, either by completing the required forms via the eRA Commons or by delegating this responsibility to another individual. A PI can access information for any grant for which they are designated the PI. See eRA Commons Roles & Privileges Matrix.

Additional Applicants
Pages 12 and 13 of the DAA must be signed by any additional person(s) who will have access to the data. Additional applicants may include those working on your project, those working in your lab, or those who have access to where the data will be stored. These individuals will be legally bound to protecting and handling the data properly. Pages 12 and 13 may be duplicated and added to the agreement to accommodate for more than 8 additional applicants. When filling out the DAA electronically, you may only include up to 8 additional applicants on your agreement.

Institutional Authority
Page 14 of the agreement must be filled out and signed by your Institutional or Administrative Authority. The institutional authority is an individual who has the authority to sign for a grant application. This individual cannot be the same as the Principal Investigator that signed on page 11, as this additional signature provides a second-party authority of the institution to ensure that the institution will uphold the terms of this agreement.

Information Security Officer
On page 15 of the DAA, your institution’s Information Security Officer’s signature is required if and only if you intend to download a local copy of the data (note: you must also initial in the line below the DAU selection). This individual may go by varying job titles, such as Information Director or Chief Information Security Officer, but is the individual responsible for information security at your institution. This signature verifies that the data, once downloaded, will remain protected by your institution’s data security protocols.

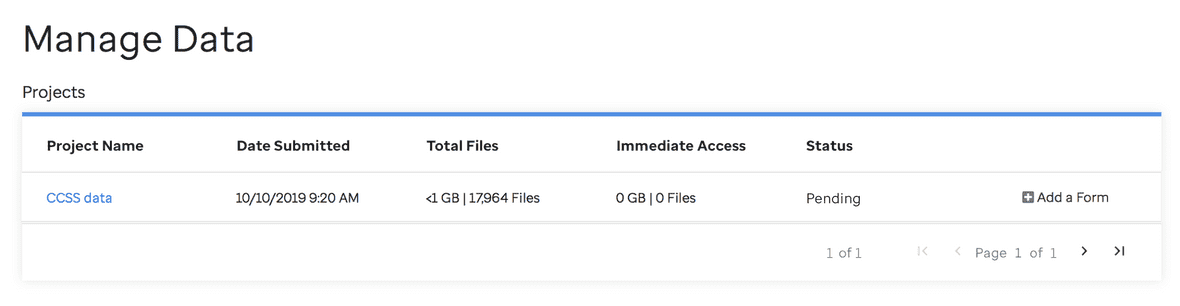
Uploading A Revised DAA
If your DAA is incomplete (for example you missed a required signature or neglected to check the box next to a dataset for which you requested data), you will be contacted by a member of the St. Jude Cloud team. Once you have made the required edits, you can reupload a revised DAA through the Manage Data page by clicking on the Add a Form button.
The Electronic Data Access Agreement Process
Users who live in the United States will be given the option to complete the Data Access Agreement electronically or manually. If you elect to complete it electronically, the setup wizard will request information about you, your institution and your associates. You will also be asked to provide a detailed description of the research project in which the data you have requested will be used.
After submitting the required information, the Data Access Agreement will be sent via email to each individual entered through the setup wizard. Each individual must follow the link in their email to sign the appropriate page of the agreement via DocuSign. If any individual rejects or declines to sign the agreement, you will need to start a new data request from the data browser.
Once you start the Electronic Data Access Agreement process, you will have a draft autosaved for you on your My Dashboard page, accessible at any time. Learn how to check your Request Status
Once all signatures have been collected, your data access request will be submitted to St. Jude Cloud. A St. Jude Cloud administrator will forward your Data Access Agreement and intended use to the appropriate Data Access Committee(s) for approval. Upon approval from the DAC(s), you will receive an email with a link to the approved data, which will be hosted through DNAnexus.
Similar Topics
Making a Data Request
Renewing your Data Access
Managing Data Overview